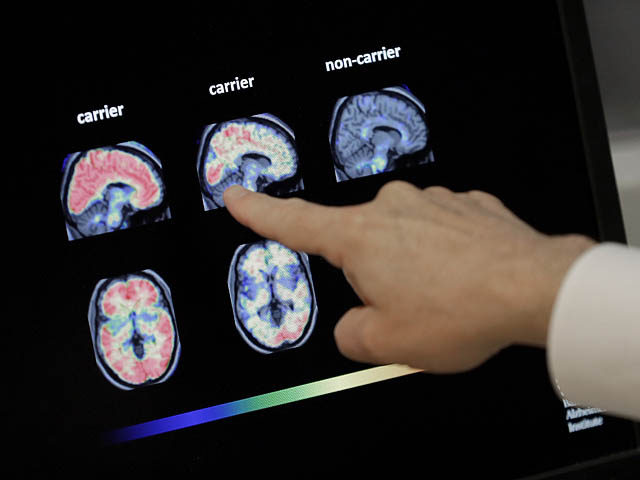
The Food and Drug Administration (FDA) has approved Eli Lilly’s drug donanemab, which slows the progression of Alzheimer’s. Eli Lilly said the drug would be sold in the United States under the brand name Kisunla and is intended for adults with early symptoms of Alzheimer’s disease. Donanemab is given as a monthly infusion, and a six-month course will cost about $12,500. In a study, a drug that targets amyloid plaques in the brain was shown to slow the progression of Alzheimer’s disease by 35% over 18 months compared to a placebo. Radio Kan Bet reported that the new Eli Lilly drug is under review by the Israeli Health Ministry and is expected to be included in Israel’s “drug basket” in 2025 or 2026.
Furthermore, there are several articles listed in the reference section related to various topics such as open data, shoe brands, online gambling, photography, and cultural discoveries. These articles cover a wide range of subjects and provide valuable information to readers interested in learning more about these areas.
Overall, the approval of Eli Lilly’s drug donanemab marks a significant advancement in the treatment of Alzheimer’s disease. The drug’s ability to slow the progression of the disease offers hope to patients and their families facing this challenging condition. As research continues to evolve in this field, it is crucial to stay informed about the latest developments and breakthroughs in Alzheimer’s treatment.